Taking a Closer Look at Soil
For their growth, plants need energy, water and nutrients. The current scientific consensus acknowledges 16 to 22 essential elements for plant nutrition. Most of a plant’s biomass is comprised of C (~45%), O2 (~42%) and H (~6%). Mineral elements (e.g. Ca, Mg, S, N, P, K, Zn, Mn, Cu, Fe, B, Na, Mo) only account for about 7% (Bergmann 1993, Schilling 2000).
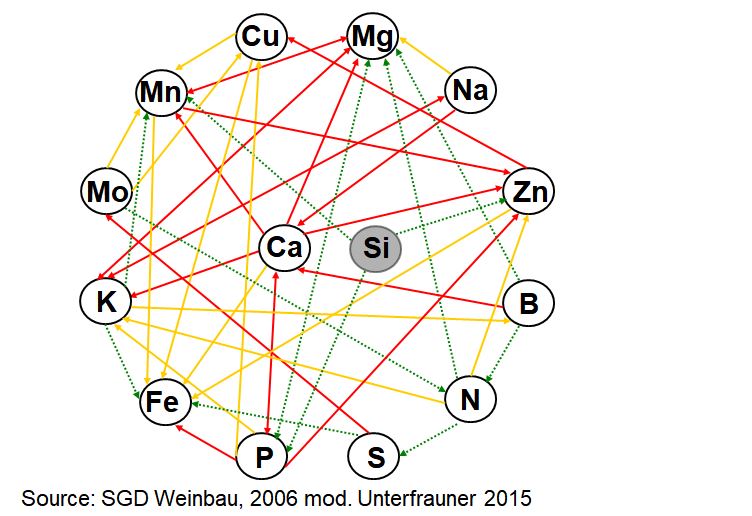
The main part of mineral nutrients is absorbed from the soil by roots, more specifically from the soil solution. During the absorption, the dissolved elements can mutually hinder or boost each other, or behave indifferently (see figure “interactive structure”).
Interactive structure of elements (mod. from SGD Weinbau, 2006)
red arrows: strong antagonism,
yellow arrows: weak antagonism,
green arrows: synergism
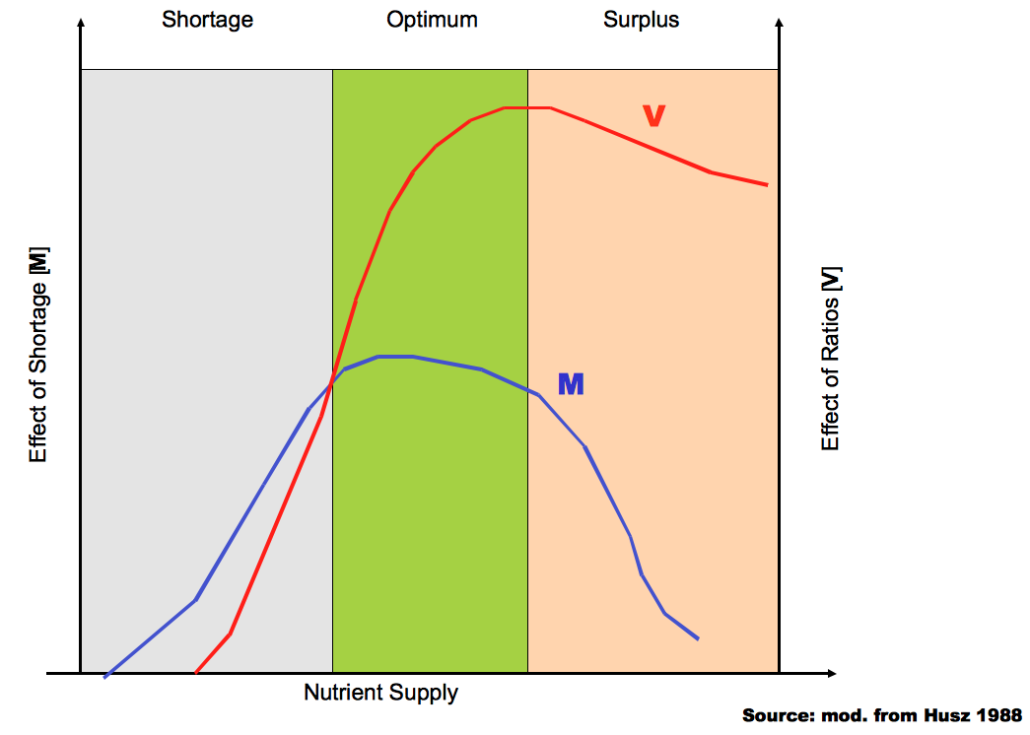
It is a well-known fact that above a certain nutrient level, the ratios of certain elements to each other are much more important for crop development than their absolute concentration (see figure). It can be assumed that more than 90% of Western Europe soils exceed that level by far.
Influence of nutrient amounts and nutrient ratios on crop development (Husz, 1988)
Nutrient Provision of the Soils
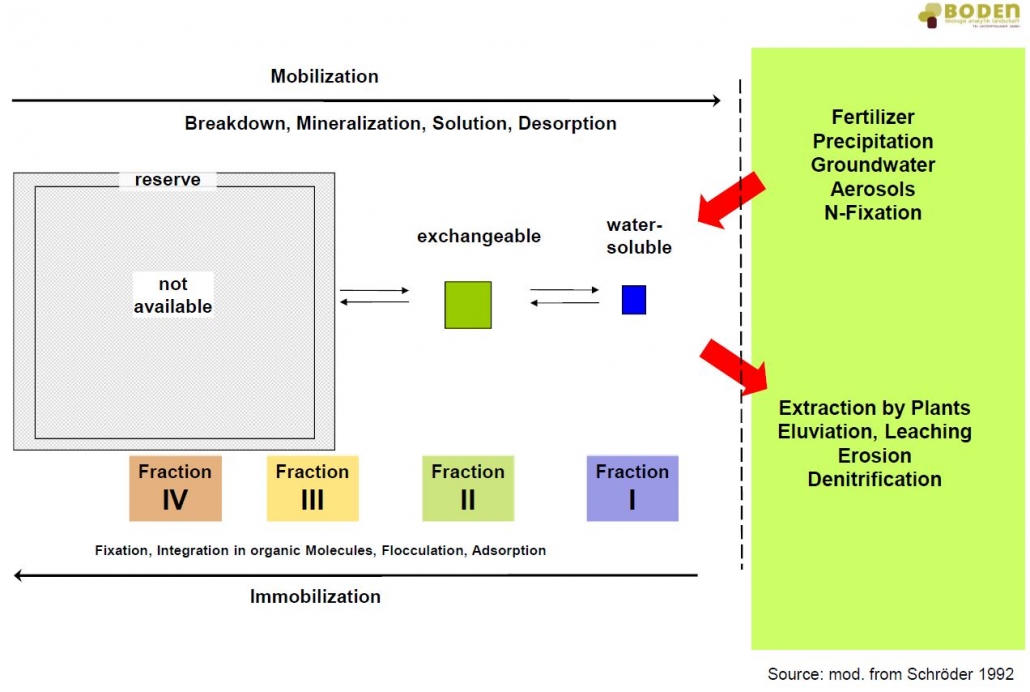
Based on the geological parent material, a soil can provide the entirety or only parts of the nutrients required by a plant in sufficient quantity. The supply of nutrients in the soil is determined by the overall available nutrients, the nutrient concentration in the soil solution, the subsequent delivery rate of nutrients and the diffusion rate. The latter one includes the mass fluxes of ions to the plant roots, which vary from soil to soil. Moreover, pedogenic parameters like soil texture (content of sand, silt and clay), concentration of humus, biological activity and characteristics of sorption play an important role. Tillage, crop rotation, type of cultivation and fertilization can directly influence the microbiological activity and with that the dynamic processes in the soil (see figure). Strongly sorbed elements can be mobilized, highly concentrated elements in solution can be converted into steadier bonds.
Dynamic equilibrium and element pools of the open system soil (mod. from Schröder, 1992)
Soil Testing / „Fractionated Analysis“
A pedological laboratory should meet the modern needs of agriculture by adequately depicting the kinetic-dynamic processes mentioned above. It is therefore necessary to “integrate nature” into the analytical process and apply scientific methods which enable a close simulation of the specific conditions of the site.
To meet these requirements, we use the “Fractionated Analysis “, a method co-developed by TB Unterfrauner which is a standardized procedure since 2004 (ÖNORM S2122-1).
Types of chemical Bonds and Stages of Availability
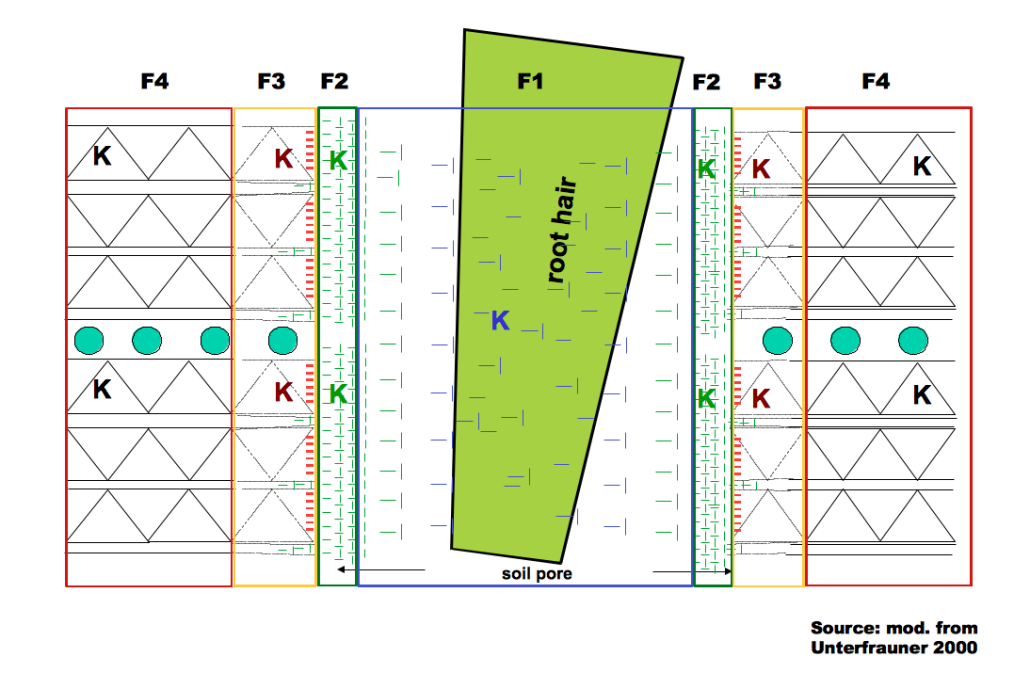
The “Fractional Analysis“ considers the different forms of chemical bonds of the elements in the soil. Depending on the form of binding, the examined elements are available to the plant either in a higher or lower degree. The figure below shows the different availability stages for the example of potassium (K). Due to reasons of simplification, the figure is limited to mineral bond types.
K can be associated to structural elements exhibiting extremely strong bonds and consequently this fraction plays no role for current as well as mid-term plant nutrition (F4 – fraction 4).
As the distance to the inner zone increases, the weathering processes accelerate. Fragments / molecules / ions from the weathered edge areas can become relevant for plant nutrition within the upcoming 10 to 15 years (F3 – fraction 3).
The surfaces of the clay minerals are charged negatively and form the so-called “sorption complex“ with organic matter. In nature, free charge differences cannot exist, so substances charged in a contrary way are always attached to each other like magnets. This accretion is not a constant (ion)-bond, the attached elements (e.g. nutrients) can be exchanged by other elements (e.g. by root excretion) (F2 – fraction 2). The “exchangeable cations“ create one of the most important pools for the nutrition of plants and microorganisms and are furthermore important for aggregate stability.
As the distance to the sorption complex increases, its magnetic effect decreases. Gradually, a zone arises where particles charged positively or negatively occur in equilibrium. Their composition, respectively the relation of the solute elements, is of greatest importance. Plant roots are only able to absorb solute substances (exceptions are little fragments of amino acids and the process of endocytosis).
Schematic display of varying substance bond intensity in the soil
Nutrient Proportions
In order to achieve optimum nutrition, the soil solution should hold an ideal composition of substance concentrations, substance ratios and substance species. There is no surplus of elements without a simultaneous lack of others! E.g. a surplus of K implies a simultaneous lack of Ca, Mg, (N).
Substances like for example the ion pairs NO3/Cl, SO4/MoO4, Mg/Mn heavily interfere each other’s absorption because of their chemical analogy. For instance, a high concentration of SO4 entails a decreased absorption of MoO4. Subsequentially, the protein metabolism is heavily disturbed because Mo is the central atom for the processes of nitrogenase and nitrate reductase.
Root exudates can only operate selectively in a limited way. The excreted acid exchanges substances from the sorption complex, those substances subsequently merge into the soil solution and rinse around the plant roots where they can be absorbed.
References
Bergmann (1993). Ernährungsstörungen bei Kulturpflanzen. 3. Auflage, Gustav Fischer Verlag.
Fachbeirat für Bodenfruchtbarkeit und Bodenschutz beim Bundesministerium für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft (BMLFUW) (2003). Richtlinien für die sachgerechte Düngung im Weinbau.
Husz (1974). Standortuntersuchung als Grundlage einer agrarökologischen Produktionsplanung. Habilitationsschrift, Univ. für Bodenkultur.
Schilling (2000). Pflanzenernährung und Düngung. Verlag Eugen Ulmer.
Scheffer-Schachtschabel (2010). Lehrbuch der Bodenkunde. 16. Auflage. Spektrum Akademischer Verlag.
Schröder (1992). Bodenkunde in Stichworten. Gebrüder Borntraeger Verlagsbuchhandlung.